Your dental lab's website content and your FDA registration could be sending mixed signals to FDA.
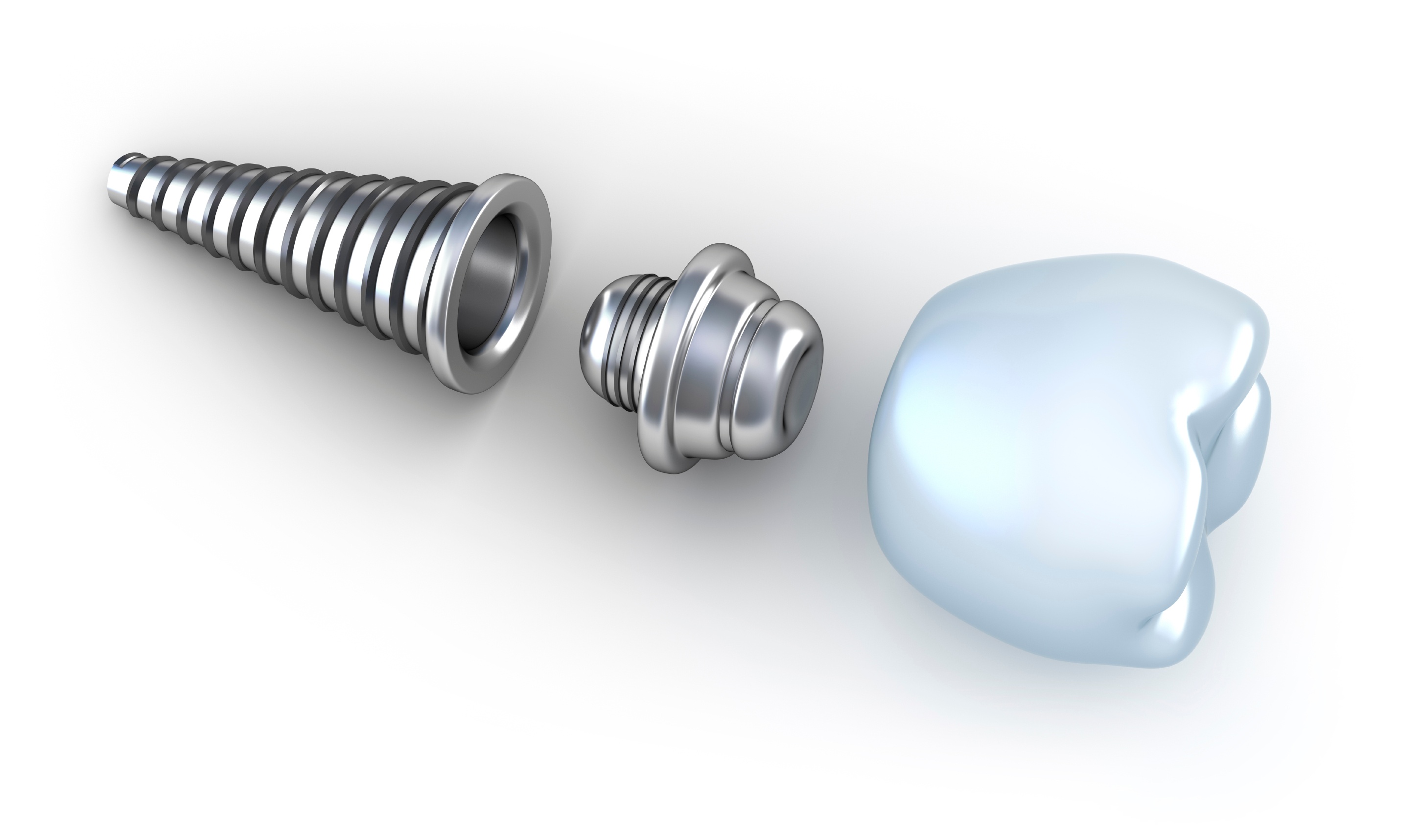
Dental labs are receiving notices from FDA asking for additional information on products being manufactured by the dental lab, such as, manufacturing custom abutments or products that are branded. FDA is looking for a 510(k) clearance in their records on FDA dental Class 2 devices and if they cannot find it the lab will receive a letter asking for the clearance information.
Have you considered whether or not the FDA registration of your foreign exporter is correct? If not, their registration could be putting your lab at risk of inaccurate registration. If you are doing business with a foreign exporter and they are not registered correctly, it can affect your FDA registration. In an FDA inspection FDA may presume you are importing something you’re not. Take an online FDA course to assist you in evaluating this part of who you are doing business with.
Get help with FDA Registration
2016 was a very interesting year for the FDA and dental laboratories. FDA inspecting dental labs increased and outcomes of those inspections produced insights into where the FDA may be looking next year and years to come. From importation of devices to manufacturing certain devices such as custom implant abutments, what might dental labs expect from FDA and the impact on their specific business model?
How to Stay on Top of FDA Dental Regulations
Learn more about FDA dental compliance by taking an online course::
3 Ways Your Product Offering Can Trigger FDA's Attention
Learn more about what SafeLink Consulting can do to help your business with compliance services, including safety compliance, to meet OSHA training requirements and quality system consulting to meet FDA compliance. SafeLink Consulting assists businesses with workplace safety training, infection control training, HIPAA training online, quality systems, assessments, audits, due diligence, and more.
Industries include:
Dentistry compliance - assisting the dental practice with meeting requirements for OSHA, HIPAA, EPA, and CDC guidelines, patient safety and employee health & safety
Dental Laboratory compliance - assisting the dental lab with meeting requirements for OSHA, FDA, and CDC guidelines, employee health & safety, plus FDA requirements for lab manufacturing custom implant abutment /gmp for medical device manufacturers
Medical Device Manufacturers compliance - assisting with meeting OSHA compliance & FDA requirements, GMP - good manufacturing practices
General Industry compliance - assisting with OSHA compliance and FDA compliance as it pertains to the specific business
Beverage Industry compliance - assisting beverage businesses such as the craft brewery, winery, cidery, distillery, vintner with meeting OSHA compliance, health & safety, FDA requirements / GMP - Good Manufacturing Practices.







Leave Comment