Preventing the spread of infection is a constant duty for workers who have potential exposure to infectious materials in the workplace. Routine and complete surface cleaning and disinfection are vital, especially with the impressive survival rates of potentially harmful pathogens on high-touch surfaces present in dental facilities.
Studies have shown that Influenza viruses can survive on hard surfaces for up to 48 hours, while viral particles of the highly-contagious norovirus can live on surfaces for days, and those of methicillin-resistant Staphylococcus aureus (MRSA) can even survive on surfaces anywhere from seven days to seven months.
Moving beyond surface disinfecting and requiring employees to wear PPE will only strengthen a workplace’s infection control practices. OSHA requires that employers provide their employees with appropriate PPE and that they make sure that it is either disposed of, if it is single use, or cleaned or laundered if multiple use. Employers must make sure it is repaired if necessary and stored after use.
The CDC has developed recommendations to keep staff and patients safe. They have simple directions for donning and removing personal protective equipment. One of the most important things in infection control that is often overlooked is that donning and removing PPE can itself be a source of contamination. The CDC has given guidelines to help prevent the possibility of such contamination.
Unfortunately, many times employees can unwittingly expose themselves to potentially infectious materials when removing their PPE. According to the CDC, PPE should be removed before you go into any area that is not involved in patient care. This includes removal before going into the lunchroom, lobby, etc.
Get a checklist for Infection Prevention Coordinator Responsibilities
Proper removal sequence as recommended by the CDC:
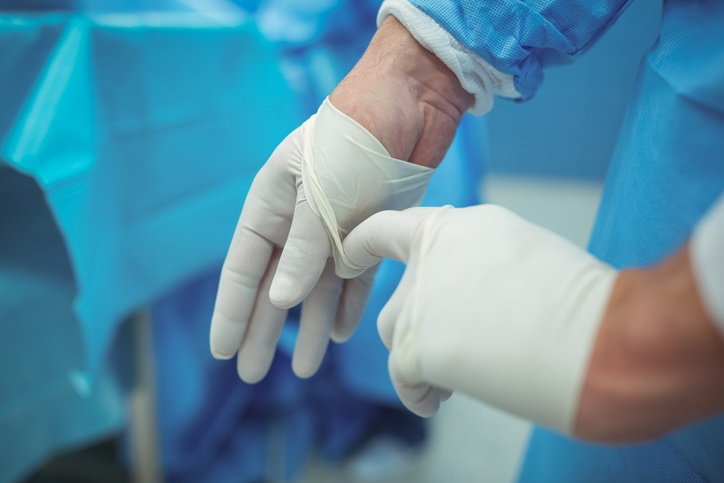
Gloves: Grasp outside of glove with opposite hand; peel off. Hold removed glove in gloved hand. Slide fingers of ungloved hand under remaining glove at wrist. Peel glove off over first glove. Discard gloves in waste container.
Tip: It is important to keep watches and jewelry off to make this process easy with removal.
Safety eyewear or face shield: Handle by headband or ear pieces. Place in designated receptacle for reprocessing or in waste container.
Tip: Clean and disinfect eye protection according to manufacturer's directions if they are not disposable.
Protective clothing: Reusable garments: Remove carefully before leaving the work area, and then perform proper hand hygiene. Disposable garments: Unfasten ties. Pull away from neck and shoulders, touching inside of gown only. Turn gown inside out. Fold or roll into a bundle and discard.
Tip: It is important to make sure that the gown does not contaminate environmental surfaces. Have a specific place for hanging the gown that is not used for hanging anything else such as patients' coats. Do not use the lunchroom or restroom for this purpose.
Mask or respirator: Grasp bottom, then top ties or elastics and remove. Discard in waste container.
Removal of personal protective equipment can be one of the riskiest areas in infection control if it is not done properly. Over spray from patient care, debris from processes, saliva, and bloodborne pathogens can cross-contaminate during the process of removal. You may be taking home those pathogens if you are not following the protocols. Reach out to SafeLink Consulting for support.
Discover more about the Importance of Properly Fitting PPE - Personal Protective Equipment.






Leave Comment